
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
/sites
/ua-mac
/raw_data
/sensor1
/timestamp
/dataset
/sensor2
...
/Level_1
/extractor1_outputs
/extractor2_outputs
...
/danforth
/raw_data
/sensor3
...
/Level_1
/extractor3_outputsAuthors:Authors: Mockler LabAuthors: Rick Ward #Data Transfer
|-terraref
| |-genomics
| | |-raw_data
| | | |-bap
| | | | |-resequencing
| | | |-ril
| | | | |-gbs
| | |-derived_data
| | | |-bap
| | | | |-resequencing
| | | | | |-danforth_center
| | | |-ril
| | | | |-gbs
| | | | | |-kansas_state# install icommands
cd $HOME
mkdir bin
cd bin
wget http://www.iplantcollaborative.org/sites/default/files/irods/icommands.x86_64.tar.bz2
tar -xvf icommands.x86_64.tar.bz2
# add icommands directory to $PATH
export PATH=$HOME/bin/icommands:$PATH
# initialize
iinit
# host name: data.iplantcollaborative.org
# port number:1247
# user name:(your Cyverse Login)
# Enter your irods zone:iplant
# iRODS password:*******
icd /iplant/home/shared/terraref/raw_data/hudson-alpha/
## transfer test data to iplant data store
touch checkpoint-file
iput -P -b -r -T --retries 3 -X checkpoint-file test_data/


traits::POST /api/datasets/uploadToDataset/<dataset id> provide file(s) and metadata{ "user": <globus\_username>
"globus\_id": <Task ID>
"contents": {
<dataset1>: {
<filename1>: {
"name": <filename1>,
"md": <file\_metadata1>
},
<filename2>: {"name": ..., "md": {...}},
<filename3>: {...},
...
},
<dataset2>: {
<filename4>: {...},
...
},
...
}
}
}git clone https://github.com/terraref/computing-pipeline.git
cd computing-pipeline/scripts/environmental_logger
git checkout masterbwa index –a bwtsw Sbicolor_313_v3.0.fasamtools faidx Sbicolor_313_v3.0.fajava –jar picard.jar CreateSequenceDictionary R=Sbicolor_313_v3.0.fa O=Sbicolor_313_v3.0.dictbbduk2 in=SampleA_R1.fastq in2=SampleA_R2.fastq out=SampleA_R1.PE.fastq.gz \
out2=SampleA_R2.PE.fastq.gz k=23 mink=11 hdist=1 tpe tbo qtrim=rl trimq=20 \
minlen=20 rref=adapters_file.fa lref=adapters_file.fabwa mem –M \
–R “@RG\tIDSAMPLEA_RG1\tPL:illumina\tPU:FLOWCELL_BARCODE.LANE.SAMPLE_BARCODE_RG_UNIT\tLB:libraryprep-lib1\tSM:SAMPLEA” \
Sbicolor_313_v3.0.fa SampleA_R1.PE.fastq.gz SampleA_R2.PE.fastq.gz > SAMPLEA.bwa.samSamtools view –bS SAMPLEA.bwa.sam | samtools sort - SAMPLEA.bwa.sortedjava –Xmx8g –jar picard.jar MarkDuplicates MAX_FILE_HANDLES_FOR_READ_ENDS_MAP=1000 \
REMOVE_DUPLICATES=true INPUT=SAMPLEA.bwa.sorted.bam OUTPUT=SAMPLEA.dedup.bam \
METRICS_FILES=SAMPLEA.dedup.metricssamtools index SAMPLEA.dedup.bamjava –Xmx8g –jar GenomeAnalysisTK.jar –T RealignerTargetCreator \
–R Sbicolor_313_v3.0.fa –I SAMPLEA.dedup.bam –o SAMPLEA.realignment.intervalsjava –Xmx8g –jar GenomeAnalysisTK.jar –T IndelRealigner –R Sbicolor_313_v3.0.fa \
–I SAMPLEA.dedup.bam –targetIntervals SAMPLEA.realignment.intervals –o SAMPLEA.dedup.realigned.bamjava –Xmx8g –jar GenomeAnalysisTK.jar –T HaplotypeCaller –R Sbicolor_313_v3.0.fa \
–I SAMPLEA.dedup.realigned.bam --emitRefConfidence GVCF --pcr_indel_model NONE \
-o SAMPLEA.output.raw.snps.indels.g.vcfjava –Xmx8g –jar GenomeAnalysisTK.jar –T CombineGVCFs –R Sbicolor_313_v3.0.fa \
-V SAMPLEA.output.raw.snps.indels.g.vcf --variant SAMPLEB.output.raw.snps.indels.g.vcf\
-V SAMPLEC.output.raw.snps.indels.g.vcf -o SamplesABC_combined_gvcfs.vcf
java –Xmx8g –jar GenomeAnalysisTK.jar –T CombineGVCFs –R Sbicolor_313_v3.0.fa \
--variant SAMPLED.output.raw.snps.indels.g.vcf -V SAMPLEE.output.raw.snps.indels.g.vcf \
-V SAMPLEF.output.raw.snps.indels.g.vcf -o SamplesDEF_combined_gvcfs.vcfjava –Xmx8g –jar GenomeAnalysisTK.jar –T GenotypeGVCFs –R Sbicolor_313_v3.0.fa \
-V SamplesABC_combined_gvcfs.vcf -V SamplesDEF_combined_gvcfs.vcf -o all_combined_Genotyped_lines.vcfjava –Xmx8g –jar GenomeAnalysisTK.jar –T VariantFiltration –R Sbicolor_313_v3.0.fa \
-V all_combined_Genotyped_lines.vcf -o all_combined_Genotyped_lines_filtered.vcf \
--filterExpression "QD < 2.0" --filterName "QD" --filterExpression "FS > 60.0" \
--filterName "FS" --filterExpression "MQ < 40.0" --filterName "MQ" --filterExpression "MQRankSum < -12.5" \
--filterName "MQRankSum" --filterExpression "ReadPosRankSum < -8.0" --filterName "ReadPosRankSum"vcftools --vcf all_combined_Genotyped_lines_filtered.vcf --min-alleles 2 --max-alleles 2 \
--out all_combined_Genotyped_lines_vcftools.filtered.recode.vcf --max-missing 0.2 --recodetassel-5-standalone/run_pipeline.pl -Xms75G -Xmx265G -SortGenotypeFilePlugin \
-inputFile all_combined_Genotyped_lines_vcftools.filtered.recode.vcf \
-outFile all_combined_Genotyped_lines_vcftools.filtered.recode.sorted.vcf -fileType VCFtassel-5-standalone/run_pipeline.pl -Xms75G -Xmx290G -fork1 -vcf \
all_combined_Genotyped_lines_vcftools.filtered.recode.sorted.vcf -export -exportType Hapmap -runfork1Database Platform: PostgreSQL
Instance: localhost
Authentication Type: Database authentication
User name: viewer
Password: DelchevskoOro
Database: select bety (if everything else is correct)ssh <login>@bety6.ncsa.illinois.edussh -L 5432:localhost:5432 <login>@bety6.ncsa.illinois.edussh -Nf -L 5432:localhost:5432 <login>@bety6.ncsa.illinois.edupgsql2shp -f terra_plots.shp -h localhost -u bety -P bety bety \
"SELECT sitename, geometry FROM sites"
ay = 3659974.971; by = 1.0002; cy = 0.0078;
ax = 409012.2032; bx = 0.009; cx = - 0.9986;
Mx = ax + bx * Gx + cx * Gy
My = ay + by * Gx + cy * GyGx = ( (My/cy - ay/cy) - (Mx/cx - ax/cx) ) / (by/cy - bx/cx)
Gy = ( (My/by - ay/by) - (Mx/bx - ax/bx) ) / (cy/by - cx/bx)Latitude: Uy = My - 0.000015258894
Longitude: Ux = Mx + 0.000020308287"gantry_system_variable_metadata": {
"time": "08/17/2016 11:23:14",
"position x [m]": "207.013",
"position y [m]": "3.003",
"position z [m]": "0.68",
"speed x [m/s]": "0",
"speed y [m/s]": "0.33",
"speed z [m/s]": "0",
"camera box light 1 is on": "True",
"camera box light 2 is on": "True",
"camera box light 3 is on": "True",
"camera box light 4 is on": "True",
"y end pos [m]": "22.135",
"y set velocity [m/s]": "0.33",
"y set acceleration [m/s^2]": "0.1",
"y set decceleration [m/s^2]": "0.1"
},"sensor_fixed_metadata": {
"cameras alignment": "cameras optical axis parallel to XAxis, perpendicular to ground",
"optics focus setting (both)": "2.5m",
"optics apperture setting (both)": "6.7",
"location in gantry system": "camera box, facing ground",
"location in camera box x [m]": "0.877",
"location in camera box y [m]": "2.276",
"location in camera box z [m]": "0.578",
"field of view at 2m in X- Y- direction [m]": "[1.857 1.246]",
"bounding Box [m]": "[1.857 1.246]",
},"sensor_fixed_metadata": {
"location in gantry system": "camera box, facing ground",
"location in camera box x [m]": "0.480",
"location in camera box y [m]": "1.920",
"location in camera box z [m]": "0.6",
},"sensor_fixed_metadata": {
"location in gantry system": "camera box, facing ground",
"location in camera box x [m]": "0.35",
"location in camera box y [m]": "2.62",
"location in camera box z [m]": "0.7",
},"sensor_fixed_metadata": {
"location in gantry system": "camera box, facing ground",
"location in camera box x [m]": "0.877",
"location in camera box y [m]": "1.361",
"location in camera box z [m]": "0.520",
"field of view x [m]": "1.496",
"field of view y [m]": "1.105",
},"sensor_fixed_metadata": {
"location in gantry system": "top of gantry, facing up, camera box, facing ground",
"location in camera box x [m]": "0.33",
"location in camera box y [m]": "2.50",
},"sensor_fixed_metadata": {
"location in gantry system": "top of gantry, facing up, camera box, facing ground",
"location in camera box x [m]": "0.400",
"location in camera box y [m]": "2.470",
},"sensor_fixed_metadata": {
"location in gantry system": "camera box, facing ground",
"location in camera box x [m]": "0.877",
"location in camera box y [m]": "2.325",
"location in camera box z [m]": "0.635",
"field of view y [m]": "0.75",
"optics focal length [mm]": "25",
"optics focus apperture": "2.0",
},INSERT INTO sites (sitename, geometry) VALUES ( 'MAC Field Scanner Field Plot 1 Season 2',
ST_GeomFromText('POLYGON((-111.975049874375 33.0745312921391 353, -111.975033517034 33.0745313124814 353,
-111.975033529346 33.0745670737771 353, -111.975049886694 33.0745670534346 353, -111.975049874375
33.0745312921391 353))', 4326));[
{
"geometry":{
"type":"Point",
"coordinates":[
33.0745666667,
-111.9750833333,
0
]
},
"start_time":"2016-08-30T00:06:24-07:00",
"type":"Feature",
"end_time":"2016-08-30T00:10:00-07:00",
"properties":{
"precipitation_rate":0.0,
"wind_speed":1.6207870370370374,
"surface_downwelling_shortwave_flux_in_air":0.0,
"northward_wind":0.07488770951583902,
"relative_humidity":26.18560185185185,
"air_temperature":300.17606481481516,
"eastward_wind":1.571286062845733,
"surface_downwelling_photosynthetic_photon_flux_in_air":0.0
}
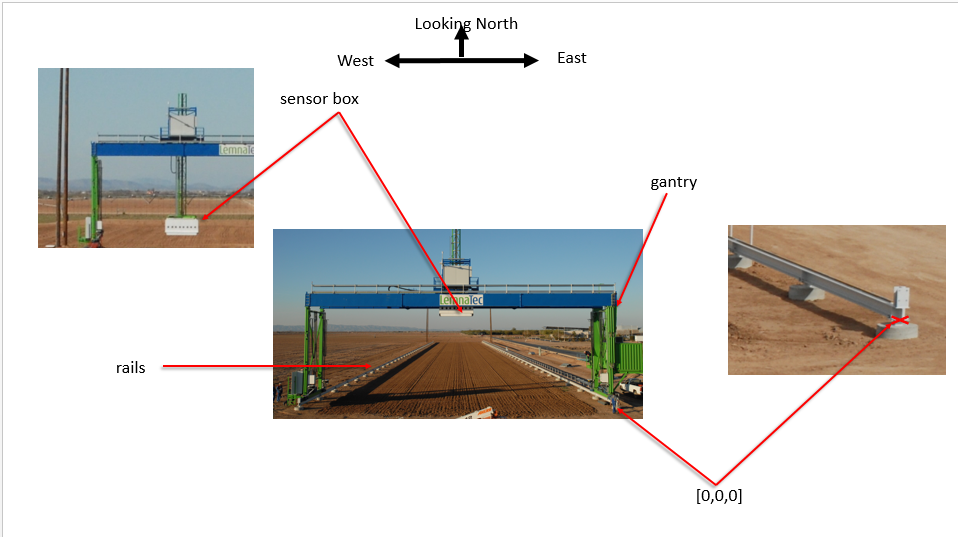
},
"variable": {
"created_at": "2016-03-07T11:23:58-06:00",
"description": "",
"id": 604,
"label": "",
"max": "1000",
"min": "0",
"name": "NDVI",
"notes": "",
"standard_name": "normalized_difference_vegetation_index",
"standard_units": "ratio",
"type": "",
"units": "ratio",
"updated_at": "2016-03-07T11:26:07-06:00"
}[Term]
id: TO:0000019
name: seedling height
def: "Average height measurements of 10 seedlings, in centimeters from the base of the shoot to the tip of the tallest leaf blade." [IRRI:SES]
synonym: "SH" RELATED []
is_a: TO:0000207 ! plant height